Influential Elements in Milk Powder Rehydration encompass factors such as particle size, density, porosity, attrition, humidity, and temperature.
Milk Powder Rehydration
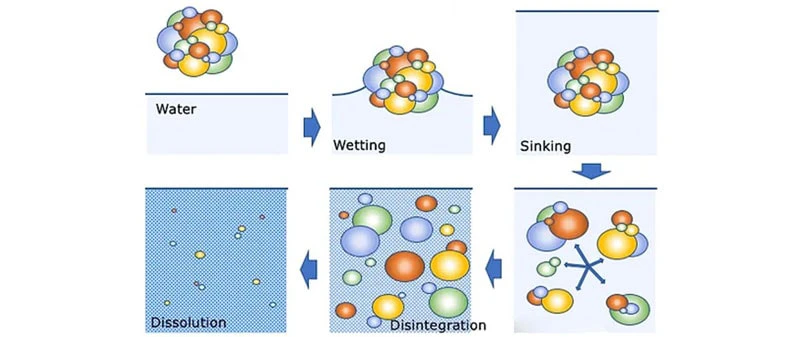
The ability of a powder to rehydrate in water is a crucial characteristic for consumers of milk powders. The rehydration of milk powder typically is governed by four key parameters: wetting, involving the penetration of liquid into the powder; sinking, where powder particles submerge into the liquid; dispersion, referring to the even distribution of powder particles; and dissolution, applicable to soluble particles as they completely dissolve.
Wettability in powder is defined as the time required for a specified amount of powder to achieve complete wetting when poured into water at a specified temperature. Occasionally, the term “sinking” is included, signifying the powder’s ability to immerse itself in water after becoming wet. Commercial milk powders typically exhibit wettability ranging from 24 seconds for skim milk powder to 120 seconds for whole milk powder. The dispersion of commercial milk powder varies between 90% for WMP and 95% for SMP. Wettability reflects a molecular interaction between the solid phase and water, representing the powder particles’ capacity to overcome water’s surface tension.
Dispersibility is the powder’s capacity to disperse into individual particles with gentle mixing when introduced into water. In the assessment, a powder sample with a known water content is evenly distributed over the surface of 25°C water. Following brief manual stirring, a portion of the mixture is filtered through a sieve, and the total solids content of the collected liquid is measured. Dispersibility is then calculated using the mass of the test portion along with the values for water content and total solids.
The Solubility Index provides an overall measurement of the ability of a powder to dissolve in water. It is defined as the volume of sediments in ml after centrifuging. The powder is dissolved in water at a certain temperature and centrifuged. The supernatant is removed and replaced by water and is centrifuged again before reading the volume of insoluble residue. The schematic of powder reconstitution is shown in the figure below.
Factors Impacting Milk Powder Rehydration
Reconstitution is influenced by various factors, encompassing particle surface characteristics, particle size and distribution, density, porosity (the internal empty space within particles), the presence of surface-active agents, particle hardness, attrition, humidity, temperature, fat and protein content, homogenization, drying methods (roller or spray), spray dryer settings (including nozzle air pressure and rotation speed), as well as meticulous sieving and application of an appropriate agglomeration method, which is briefly outlined below.
Particle Size effect on milk powder rehydration
Various methods are employed to measure milk powder, including microscopy, image analysis, sieving, and light scattering. Typically, liquid milk particles are below 2-3 µm in size, while powdered milk particles range from 100 µm to 250 µm on average, about 40 to 100 times larger. For whole milk powder, the D50 falls between 142 µm to 149 µm, while for skim milk powder, it ranges from 121 µm to 126 µm, indicating that whole milk powder has a larger average particle size than skimmed milk powder.
(D50, or median particle diameter, signifies the size at which 50% of the particles are larger, and 50% are smaller.)
Large particles and agglomerates typically have diameters ranging from 200 µm to 500 µm, while fine particles are smaller than 125 µm. An optimal reconstitution particle size falls between 150 µm and 200 µm, indicating improved dispersion and absorption.
Particle size, measured in micrometers (µm), plays a crucial role in reconstitution. Laser diffraction systems are advanced techniques for precise particle size analysis, revealing that regular skim milk powders have an average size of 85 µm, while fat-containing powders range from 230 µm to 250 µm. Dispersion is influenced by particle size and morphology. Smaller particles or a higher percentage of fine particles can result in poorer flowability, dispersion, and longer wetting times. Powders with sizes above 200 µm are considered free-flowing, while fine powders face cohesion challenges, leading to increased resistance and difficulty in flowing.
Agglomeration effect on milk powder rehydration
The primary goal of agglomeration is to combine small particles into larger ones. Agglomerated powders consistently exhibit higher average particle sizes compared to non-agglomerated (regular) powders. When powders are excessively fine, they tend to form lumps, impeding the dissolution rate. Agglomeration alters the porosity of the powders and facilitates water penetration into the granules.
To achieve particle agglomeration, it is necessary to increase the size of fine particles, typically ranging from 30 to 50 microns, to a range of 150 to 200 microns. This transformation enhances flowability, dispersion, and wettability, while also minimizing dust pollution. Granulation or agglomeration substantially boosts powder dispersion from 41% to 62% under rewetting conditions, thereby improving dispersion, flowability, and wettability.
Porosity effect on milk powder rehydration
Materials with finer grains generally exhibit greater porosity compared to those with larger grains. In the illustration below, small particles in poorly-grained materials occupy the spaces between larger particles, resulting in a significant decrease in porosity and hydraulic conductivity. (In the image, black indicates solid materials, while blue represents empty space.)
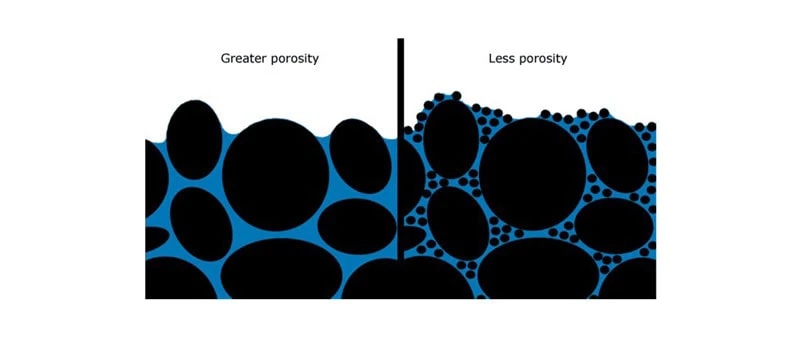
Increased porosity and larger pore size enhance wettability, while the presence of surface-free fat and a reduction in interstitial air volume (porosity) constrain and decelerate the rehydration process of milk powder in water.
Protein effect on milk powder rehydration
In comparison to other milk powders, protein powders often exhibit inferior rehydration and dispersing properties. High-protein dairy powders, including isolates, tend to have suboptimal flowability because of their cohesive nature. This poses challenges for manufacturers and end consumers in further processing these powders.
Carbohydrate effect on milk powder rehydration
Products with higher carbohydrate content exhibit improved wetting, requiring less time for soaking in water.
Temperature effect on milk powder rehydration
Dispersion improves with increased temperature. Observations indicate that water temperature at 60°C yields better dispersion results compared to 40°C and 24°C. Additionally, the solubility of proteins in milk powder may vary based on the reconstitution temperature, with higher temperatures leading to greater solubility.
Fat effect on milk powder rehydration
Test results indicate that the solubility of skim milk powder surpasses that of full-fat powder due to reduced dispersion of fat in water. The outcome is influenced by factors such as the type of milk powder, spray dryer parameters, temperature, humidity, and other conditions.
Density effect on milk powder rehydration
Finer particles are associated with higher loose bulk density and tapped bulk density. The loose density of full-fat dry milk is lower than that of skim milk. Coarse particle samples consistently exhibit the lowest bulk density, while fine particle samples demonstrate the highest bulk density. This is attributed to the irregularity of coarse particles compared to medium and fine particles. Fine particles, being more spherical, contribute to higher bulk density due to lower interstitial air content, while irregular particles lead to lower bulk density. It is concluded that maximum solubility through dispersion is achieved with milk powders having a bulk density of less than 0.4 g/ml. Higher bulk density results in reduced dispersion.
Humidity effect on milk powder rehydration
Moisture content plays a crucial role in flowability, storage, and overall quality, influencing milk solids’ solubility. To maintain optimal solubility (between 15 and 38 percent moisture content), moisture levels are carefully regulated throughout the process. Full-fat milk powder tends to have lower moisture content than skim milk powder. Elevated humidity levels can increase particle stickiness, leading to tight agglomeration and lumpy states, ultimately reducing solubility. High humidity results in compact particle clusters, while low humidity causes loose aggregation. It is essential to manage moisture levels to maintain a granular powder structure.
Drying Method effect on milk powder rehydration
The solubility of spray-dried skimmed milk powder (SMP) and whole milk powder (WMP) typically exceeds 99%, whereas roller-dried milk powder tends to have lower solubility (around 85%). Particle shape and size are influenced by the drying method and milling operation. Spray-dried powder particles are spherical with diameters ranging from 10 to 250 microns, primarily determined by the nozzle properties. In contrast, roller-dried powders exhibit a compact structure, irregular shape, and lack blocked air, with final particle dimensions more influenced by the milling process. The size and distribution of spray-dried product droplets are heavily dependent on the type of nozzle used for milk nebulization. Roller-dried powder generally has a larger average particle size of approximately 150 microns and a low vacuole volume, while spray-dried powder has a smaller particle size of about 70 microns and a high vacuole volume.
Homogenization effect on milk powder rehydration
Effective homogenization is crucial for breaking down large fat particles during the production process. A substantial amount of fat particles binds to the outer layers of protein particles, forming larger aggregates. Inadequate homogenization limits the stability and flowability of powdered milk. Conversely, excessive homogenization results in a significant increase in the specific surface area of fat particles, leading to the flocculation of milk powder or hastening the oxidation of fat. This, in turn, diminishes the taste and stability of the product. Therefore, continuous monitoring of milk powder particle size changes is an integral part of the production process.
Attrition effect on milk powder rehydration
The susceptibility to attrition is influenced by the chemical composition of the material and the strength and shape of its particles. Round particles typically exhibit less attrition compared to jagged, irregular particles, as the latter’s edges are more prone to breakage.
Conclusion
the rehydration of milk powder is a nuanced process, involving stages like wetting, sinking, dispersing, and dissolution. Factors such as particle size, density, porosity, and others significantly influence the outcome. From understanding the molecular interactions to exploring the impact of drying methods, homogenization, and more, it’s evident that achieving optimal rehydration is a complex interplay of diverse elements.
For inquiries or to contact the Hiroland, please refer to the Contact section.
Read More: Exploring the World of Buttermilk Powders: A Comprehensive Comparison
Read More: Particle size distribution applied to milk powder rehydration













2 thoughts on “Influential Elements in Milk Powder Rehydration”
Fine waay of telling, and nice post too obtain data concerning my presentation subject, which i am going to preent in academy. https://www.Waste-ndc.pro/community/profile/tressa79906983/
Muchas gracias. ?Como puedo iniciar sesion?